I always assume badflash is super human..., but that is sound advice. That goes for any concentrated acid too. I'm sure he was going to wear goggles, gloves, close toed shoes, pants, and long sleeves, maybe even a lab coat while he was sloshing around these chemicals!
Results 11 to 20 of 26
Thread: Fish Emulsion
-
05-31-2011, 02:58 PM #11
Re: Fish Emulsion
-
05-31-2011, 03:34 PM #12
Re: Fish Emulsion
I wasn't worried about badflash, I was worried about you.

-
05-31-2011, 03:41 PM #13
Re: Fish Emulsion
 Originally Posted by cedarswamp
Originally Posted by cedarswamp


 I see what you did there!
I see what you did there!
-
06-01-2011, 08:51 AM #14
Re: Fish Emulsion
HCL + NaOH==> H2O+ NaCL. That is salt and won't be released. KOH ends is the KCl. Both end up with ionic chlorides nd are bad for plants.
H2SO4 +NaOH==> H2O +NaSO4. Probably less of an issue, but Sulfuric acid is really nasty stuff. Phosphoric Acid is far safer. Use of Phosphoric Acid also maintains organic certification. I can't say where you would be at with other acids.The best fertilizer is the farmer's shadow
-
06-01-2011, 10:06 AM #15
Re: Fish Emulsion
Hydrochloric acid also maintains organic certification. Originally Posted by badflash
Originally Posted by badflash
Those are correct in that they are balanced and the right resulting reaction, but that is not what would happen in your situation:
Step 1
HCl + H2O => H3O + Cl-
Step 2
............(Chlorine volatilizes)
H3O (aq) + Cl- (aq) => H3O (aq) <=> H2O (l) + H+ (aq)
Step 3
H3O (aq) + NaOH (aq) => 2H2O (l) + Na+ (aq)
That is what happens if you allow some time before adding the base (neutralizer); the chlorine will volatilize (off gas). As I mentioned, you can also boil the solution to be 100% sure there is none left since chlorine has a 0 solubility in aqueous solutions at 100 C/212 F. Remember, HCl is a strong acid. A strong acid is an acid that ionizes completely in an aqueous solution. Your formula below is only applicable to your situation in the absence of time: HCl + NaOH => H2O+ NaCl
As far as the sulfuric acid, I didn't think it would be an issue either (I thought you would have calcium though). The plants will absorb both elements, and under certain conditions may even absorb large quantities of the sodium (Na).
Do you have to use sodium hydroxide (lye) as your neutralizing agent? I thought you were using lime (Ca(OH)2), which would yield calcium based salts that are common fertilizers...
I'm not at all familiar with the process, but I would imagine the acid and base used only has to do with adjusting the pH and not adding anything BAD into the mix. This would give you a TON of options, in some cases cheaper! I know you want to keep it organic; so, this would limit as well, but not all that much. Does that sound right or...? Originally Posted by badflash
Originally Posted by badflash
BTW, I really think this is a great idea. In fact, I was just telling my friend all about it yesterday (and your squeal quote). Sulfuric acid is very cheap (battery acid) and so is lime (calcium hydroxide, about $1 a pound local, cheaper than lye I believe). I'm sure you will figure it out, but if you want help with brainstorming some chemicals, don't hesitate to ask buddy!
-
06-01-2011, 10:19 AM #16
Re: Fish Emulsion
badflash, I forgot to even consider... but, what temperature do you think the mixture you make after adding acid will get to? Will it bake in the sun and maybe get near 100 C/212 F? If so, you really want to consider hydrochloric acid because your chlorine will really just be gone and in no time once it hits those temps (it's instant not counting the time the bubbles take to hit the surface).
Here, I'm posting a graph of what I am talking about:
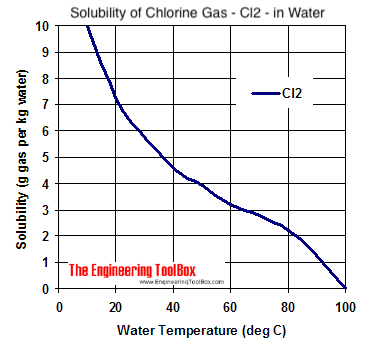
Lastly, I would always send any new substance I am using/selling as a fertilizer to my local extension's soil lab. It usually only costs a few bucks, and you can always have them check for the sodium, chlorine, or anything else you might suspect may not have behaved as theorized given the complex mixture of other organic and inorganic compounds.
-
06-02-2011, 07:12 AM #17
Re: Fish Emulsion
I dissagree with your chemistry. HCL will not produce hydrogen peroxide and free chlorine. The chlorine will not off-gas.
The best fertilizer is the farmer's shadow
-
09-02-2011, 10:48 AM #18Members

- Join Date
- Aug 2011
- Location
- Sacramento, Ca
- Posts
- 20
Re: Fish Emulsion
I was reading online about fish by product and I remembered this thread. and here is what I found online as a howto. Hope it helps anyone.
http://faq.gardenweb.com/faq/lists/orga ... 31662.html“Being happy doesn't mean that everything is perfect. It means that you've decided to look beyond the imperfections."
-
09-02-2011, 01:21 PM #19
Re: Fish Emulsion
If you try making any, be sure to use a fine screen to keep the maggots out. It gets real digusting real fast if you are not careful. Use phosphoric acid, not HCl. The results are much better. Anyone that says otherwise, post your personal results, not something that was trolled in off the internet, published papers are allowed of course.
100C is 212F, why would anyone boil fish guts? Never mind, I withdraw the question.The best fertilizer is the farmer's shadow
-
09-02-2011, 04:09 PM #20
Re: Fish Emulsion
It's 2 water molecules not hydrogen peroxide (2H2O vs. H2O2, respectively). Also, the chlorine may off gas it may react with something else. There's so much stuff in there it's hard to say, but by boiling it there is a high chance it will off gas. Of course you don't have to boil it, but when it sits outside the point is it gets very hot and approaches the same result (please see graph). The heat will either drive a reaction with the chlorine or if no reaction it will definitely off gas. It may also form organic compounds, which translates to a slow release fertilizer for the plants because, as you know, they need (Cl) as well. Originally Posted by badflash
Originally Posted by badflash
Sorry for the late response, I didn't think anyone would bother reading this... oops...





 Reply With Quote
Reply With Quote


